More about UDI
Do you want to know more and anticipate UDI regulatory developments worldwide?
Visit the Public Policy Database


What is UDI?


According to the International Medical Device Regulators Forum (IMDRF), a Unique Device Identification (UDI) system is intended to provide a single, globally harmonised system for positive identification of medical devices through distribution and use. UDI requires the label of devices to bear a globally unique device identifier captured in a data carrier (AIDC), and if applicable, its Human Readable Interpretation (HRI) on the label or the device itself. This identifier and data carrier should be based on standards from accredited issuing agencies, including GS1.
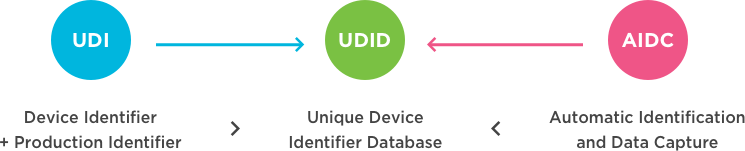
Healthcare professionals and patients will no longer have to access multiple, inconsistent, and incomplete sources to identify a medical device and, its key attributes. The Unique Device Identification Database (UDID) is a designated source for additional information.
The benefits of UDI can only be achieved if all stakeholders, from the manufacturer to healthcare providers and patients, use UDI throughout their processes and systems. Therefore, it is imperative that all stakeholders be educated about the development and use of a UDI System.
Source: IMDRF Unique Device Identification system (UDI)
Across the world, different regulators, (government) agencies and healthcare providers are aiming for a globally harmonised implementation of UDI and make patient safety a strategic priority.
UDI database GDSN mapping
The UDI database GDSN mapping section lists all the healthcare-related documents by country.
UDI and GS1
GS1 is an UDI issuing agency or Entity for many UDI regulations worldwide, including in the European Union, Brazil, China, Egypt, Saudi Arabia, Singapore, South Korea, Taiwan, Türkiye and the USA. This means that manufacturers or labelers can use the GS1 standards to comply with UDI requirements from these jurisdictions.
The GS1 system of standards provides a global framework to identify, capture and share medical device product information, thereby enabling a consistent worldwide implementation of UDI. UDI regulatory requirements have a translation into GS1 standards as shown in the table.
GS1 Member Organisations issue GS1 Company Prefixes (GCP), enabling the generation of GS1 identification keys for companies to create UDIs. They also provide direct support and training on the use of GS1 standards to implement UDI requirements worldwide.
| UDI regulatory requirements | GS1 Standards |
|---|---|
|
Basic UDI-DI Unique identifier specific to a medical device product family in the EU |
GMN (Global Model Number) No Application Identifier (AI) for regulated medical devices |
| Master UDI-DI* |
For devices currently utilising GTIN per existing (made-to-stock) GTIN AI (01) allocation rules:
For made-to-order devices not currently identified by a GTIN:
|
| NOTE: Master UDI-DI (MUDI-DI) meets a requirement for highly individualised medical devices. The first published regulatory requirement covers contact lens, per both made-to-stock (standard contact lenses per regulation (EU) 2017/745 as amended 7 October 2023) and made-to-order contact lenses. | |
|
UDI-DI * Device Identifier (DI) |
GTIN * Global Trade Item Number |
|
UDI-PI * Production Identifier (PI) (if applicable) |
AI * Application Identifier (AI)
|
| Product Identifier data will vary by medical device type and manufacturer current practice | |
| UDI-DI + UDI-PI = UDI | GTIN or GTIN + AI(s) = UDI |
| * The HRI (Human Readable Information) format shall follow the rules of the UDI Issuing Entity | |
UDI in Brazil
ANVISA released the UDI regulation establishing a UDI system applying to medical devices placed on the Brazilian’s market, entering into force on 10 January 2022.
The regulator plans a six-year rollout of UDI requirements according to risk class. This Resolution applies to all medical devices regulated by Anvisa, except for custom-made medical devices and medical devices under clinical investigation.
GS1 has been accredited by ANVISA as issuing agency for unique device identifiers (UDIs).
The requirements are aligned with the IMDRF framework.
UDI in China
The Rules for Unique Identification System for Medical Devices (hereinafter referred to as the Rules), released in August 2019 by China National Medical Products Administration (NMPA), has ushered in the stepwise implementation of Unique Identification system for medical devices.
GS1 China is a qualified issuing agency for UDI in China and GS1 standards meet the NMPA’s criteria for issuing UDIs. GS1 Member Organisations across the world will help manufacturers implement with the requirements of the NMPA UDI regulation, to support patient safety and supply chain security.
On October 14, 2019, NMPA issued the Announcement on Effective Implementation of Unique Identification for the First Batch of Medical Devices (hereinafter referred to as the Announcement), which clearly defines the scope, schedule and work requirements of unique identification for the first batch of medical devices. As per the Announcement, for medical devices listed in the first batch, the registrant shall follow the Rules, timely, orderly and effectively perform the coding of Unique Identification, and complete the submission of the registration system and database for unique identification.
On September 29, 2020, NMPA, National Health Commission and National Healthcare Security Administration issued the Announcement on Further Promoting the Pilot and Effective Implementation of Unique Identification for the First Batch of Medical Devices which extended the scope and redefined the schedule.
On September 13, 2021, NMPA, NHC and NHSA issued the Announcement on Effective Implementation of Unique Identification for the Second Batch of Medical Devices.
On February 10, 2022, NMPA, NHC and NHSA issued the Announcement on Effective Implementation of Unique Identification for the Third Batch of Medical Devices.
Useful information
English version of the Rules for Unique Device Identification System
NMPA published 5 related standards which can be found here
GS1 China UDI webpage for UDI guidance and FAQ
GS1 China Quick Guide for NMPA UDID GDSN Testing
GS1 China Filling Procedures of GS1 Company Prefix (GCP) / Global Trade Item Numbers (GTINs)
UDI in Chinese Taipei
Taiwan has begun a phased implementation of UDI, which includes both labelling and database reporting requirements. The “Requirements for Indicating the Unique Device Identifier on Medical Device Labels" came into force on 1 May 2021.
Issuing agencies include GS1, HIBCC and ICCBBA.
UDI in the EU
The EU Medical Device Regulation (MDR) and In-vitro Diagnostic Regulation (IVDR) were adopted on 5 April 2017 and define the requirements for the EU UDI system.
On 7 June 2019, GS1 was designated by the European Commission as an issuing entity for Unique Device Identifiers (UDIs). GS1 standards are enabling healthcare manufacturers from around the world to create and maintain UDI numbers by following the EU Regulations and the GS1 General Specifications.
Required product data will be submitted to EUDAMED, i.e. the EU regulatory database for regulated medical devices.
The EU Regulations have introduced a new concept: the Basic UDI-DI, that aims at grouping regulated medical devices under the same identifier. GS1 has developed a new key to support the implementation of the Basic UDI-DI: the Global Model Number (GMN). The GMN generator tool helps generate the GMN (Basic UDI-DI), calculate the related check character pair or verify your GMN (Basic UDI-DI).
The European Commission submitted a work request to GS1 to develop a Master UDI-DI for implementation of a new level of identification for specific products. For more information, and to join the Global Standards Management Process Mission Specific Work Group (GSMP MSWG) see the Call to Action.
Compliance dates for UDI requirements and complementary information by type of actor in the EU are available in the European Commission’s website in the section “Getting ready for the new regulations”.
The European Commission is working on implementation details and is regularly publishing complementary guidances and MDCG endorsed documents, including Q&As and proving guidance on specific device types and assignment rules.
Useful information
European Commission UDI Helpdesk
European Commission webpage on Medical Devices
European Commission FAQ on UDI
European Commission MDCG endorsed documents and other guidance
GS1 UDI identifier Basic UDI-DI (video)
GS1 brochure “Be ready for UDI in Europe!”
GS1 Global Model Number (GMN) generator
GS1 Call to Action: New EU requirements for medical devices identification (i.e. “Master UDI-DI”)
UDI in Egypt
According to the President of the Egyptian Drug Authority’s Decree number (499) of the Year 2021, "Medical Supplies factories, companies, distributors, importers, and warehouses with all of its types and sources that deal in the Egyptian market are committed to using the Global trade items barcode (GTIN) for all medical supplies that are being traded inside the local market, through dealing with the electronic platform (Me Device)".
According to the Guidance on Requirements for Unique Device Identification (UDI) for Medical Devices, "In case the medical device is encoded with any other issuing agencies designed by the IMDRF, the brand owner / Agent should submit a request to EDA reporting them about the issuing agency they adopted".
UDI in Saudi Arabia
The SFDA UDI regulation was enacted on 1 August 2021, and GS1 has been accredited as issuing agency. The database, SAUDI-DI, aims to document unique device codes for medical devices based on accredited international standards, allowing all stakeholders to identify medical device information through the unique device identification code registered in the system.
Manufacturers and their authorised representatives are directly responsible for providing all data of unique device identification codes for their medical devices.
Saudi_DI is available since 1 October 2020.
The UDI regulations in Saudi Arabia cover all medical devices distributed in the KSA market and manufacturers, except for custom-made devices and investigational medical devices.
The SFDA guidance outlines the main requirements for the unique identification system to be applied for medical devices allowed to be imported and marketed in Saudi Arabia.
UDI in Singapore
The Health Sciences Authority (HAS) in Singapore released the final UDI Guidance. The purpose of the guidance is to provide clarity on the regulatory requirements for Unique Device Identification (UDI) implementation in Singapore and the details on the steps to submit UDI information into the Singapore Medical Device Register (SMDR) and Class A Medical Device Database.
The fundamental elements of the UDI system in Singapore are in alignment with the internationally harmonised principles published by the International Medical Device Regulators Forum (IMDRF).
GS1 has been accredited by the HSA as issuing agency for unique device identifiers (UDIs).
Devices identified and marked according to the UDI requirements for the U.S. & the EU will be accepted by HSA. The UDI-DI will be registered in the Singapore Medical Device Register (SMDR), an online database.
UDI in South Korea
Following the publication in 2028 of notification No. 2020-29, the UDI barcode labelling obligation came into force in 2020 for Class IV devices. Requirements aligned with the IMDRF framework in which South Korea is participating.
In 2022 UDI has been fully implemented with all classes of medical devices requiring a Unique Device Identifier.
The Ministry of Food and Drug Safety (MFDS) has implemented Unique Device Identifier (UDI) database systems for submitting regulatory-mandated medical device product data. The database is called Integrated Medical Device Information System (IMDIS). The National IMDIS collects both medical device data and medical devices’ distribution (transaction) records.
GS1 has been accredited by Korean Authorities as issuing agency for Unique Device Identification (UDI).
UDI in Türkiye
In Türkiye, medical devices are regulated by the Turkish Medicines and Medical Devices Agency (TİTCK). The Unique Device Identification (UDI) requirements follow the guidelines of the European Union Medical Device Regulation (EU MDR). GS1 is an accredited issuing agency in Türkiye.
The UDI requirements for medical devices in Türkiye include:
- UDI Marking: Medical devices must have the UDI marked on their label or packaging.
- UDI Database: The TİTCK maintains a database called the Ürün Takip Sistemi-Product Tracking System (ÜTS) where manufacturers are required to register their medical devices and their UDIs.
Useful information
UDI in the U.S.
In 2013, the U.S. Food and Drug Administration (U.S. FDA) released the UDI rule. The FDA established the unique device identification system to adequately identify medical devices sold in the United States from manufacturing through distribution to patient use.
According to the U.S. FDA, when fully implemented, the label of most devices will include a unique device identifier (UDI) in human- and machine-readable form, which will ultimately improve patient safety, modernise device postmarket surveillance, and facilitate medical device innovation.
In general, the UDI final rule requires device labelers (typically, the manufacturer) to:
- Include a unique device identifier (UDI), issued under an FDA-accredited issuing agency's UDI system, on device labels, device packages, and in some instances, directly on the device.
- Submit device information to the Global Unique Device Identification Database (GUDID).
Since 17 December 2013, GS1 has been accredited by the U.S. FDA as an issuing agency for Unique Device Identification (UDI).
Useful information:
U.S. FDA UDI system dedicated webpage
U.S. FDA UDI Compliance Policies and UDI Rule Compliance Dates
U.S. FDA - UDI Rule, Guidances, Training and Other Resources
US FDA - Classify Your Medical Device
GS1 US webpage on Unique Device Identification